Description and Composition of Ziagen
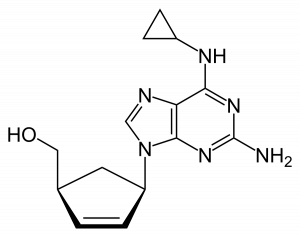
Ziagen contains Abacavir as its active pharmaceutical ingredient. Abacavir is an antiretroviral agent with ABC as its synonym. It is indicated for treatment of HIV infection in combination with other antiretroviral agents. It usually comes as tablets and solutions with strength of 300 mg and 20 mg/mL respectively.
Pharmacology of Ziagen
Ziagen is a nucleoside reverse transcriptase inhibitor (NRTI). It is converted to its active metabolite, carbovir triphosphate (CBV-TP), which inhibits the activity of HIV-1 and HIV-2 reverse transcriptase by competing with the natural substrate deoxyguanosine-5-triphosphate (dGTP) and by inserting itself into viral DNA. Resistance and cross resistance with other nucleoside reverse transcriptase inhibitors have been observed with antiretroviral therapy.
Abacavir is rapidly absorbed following oral administrating. There is no significant difference in systemic bioavailability when abacavir is administered with or without food. Metabolism of abacavir in humans does not involve cytochrome P450 enzymes. Abacavir does not significantly inhibit CYP3A4, CYP2D6, or CYP2C9. The main routes of elimination are metabolism by glucoronyl transferase and alcohol dehydrogenase to inactive metabolites. Subsequent excretion of abacavir is primarily through the urine, which accounts for 83% of the dose. The rest of the dose is excreted fecally. The mean elimination half-life (t1/2) is 1.5 hours.
Uses or Indications of Ziagen
Ziagen is used in the treatment of HIV infection in combination with at least two other antiretroviral drugs.
Dosage of Ziagen
Adults: HIV infection (in combination with at least two other antiretroviral medicines), 300 mg twice daily or 600 mg once daily.
CHILD: (3 months – 16 years): HIV infection (in combination with at least two other antiretroviral medicines), 8 mg/kg twice daily; max. 600 mg daily.
Hepatic Impairment: Abacavir is primarily metabolised in the liver. No dose recommendation can be made in patients with mild hepatic impairment. Close monitoring for abacavir toxicities recommended. Avoid use in patients with moderate to severe hepatic impairment.
Renal impairment: No dose adjustment is necessary in patients with renal impairment.
How to Use (Take) Ziagen
Ziagen is used by mouth (orally). It may administer without regard to meals. Counsel patient about the importance of regular dosing (intermittent therapy may increase sensitization). Tablets may be crushed and added to a little amount of liquid or semi-solid food for those who are not able to swallow tablets.
Contra-indications of Ziagen
It is contraindicated in those hypersensitivity to abacavir or any other ingredient of the formulation. Also, it is contraindicated in patients who are HLA-B*5701-positive. It is also contraindicated in moderate or severe hepatic impairment.
Hypersensitivity reaction: Symptoms usually appear within the first six weeks of initiation of therapy with abacavir, although these reactions may occur at any time during therapy. Monitor patients closely especially during the first two months of treatment with abacavir, with consultation every two weeks. Discontinue therapy immediately if hypersensitivity reaction occurs and abacavir must never be restarted in such patients.
Lactic acidosis: This is a rare but severe, potentially life-threatening complication associated with use of nucleoside reverse transcriptase inhibitors (NRTI). Lactic acidosis may occur after a few to several months of NRTI treatment. Patients with hyperlactataemia may be asymptomatic, critically ill, or may have non-specific symptoms such as dyspnoea, fatigue, nausea, vomiting, diarrhoea and abdominal pain. Risk factors for NRTI-related lactic acidosis include female gender and obesity. Patients at increased risk should be closely monitored clinically.
Lipodystrophy: Combination antiretroviral therapy has been associated with the redistribution of body fat (lipodystrophy) in HIV-infected patients; the evidence for abacavir as a causative agent is weak. Risk factors may include older age of the patient, longer duration of antiretroviral therapy and related metabolic disturbances.
Pancreatitis: Discontinue therapy immediately if patient shows clinical signs and symptoms of pancreatitis.
Myocardial infarction: The use of antiretroviral therapies, including abacavir, may be associated with an increased risk of myocardial infarction. Precautionary measures should be taken to minimize all modifiable CVD risk factors e.g., hypertension, hyperlipidaemia, diabetes mellitus, and smoking.
Combination therapy with abacavir: Abacavir should only be used in combination with zidovudine and lamivudine in the treatment of antiretroviral naïve patients in situations when a regimen based on a protease inhibitor (PI) or NNRTI cannot be used. Abacavir should not be used as part of a triple combination regimen including tenofovir.
Hepatic disease: Plasma concentrations of abacavir are substantially increased in patients liver disease. Therefore, the use of abacavir in patients with moderate hepatic impairment is not recommended unless judged necessary and requires close monitoring. Consider discontinuing therapy if there is evidence of worsening liver disease in such patients. For patients with severe hepatic impairment, abacavir is contraindicated Patients with chronic hepatitis B or C and treated with combination antiretroviral therapy are at an increased risk of severe and potentially fatal hepatic adverse events. Consult individual product literature if concomitant antiviral therapy for hepatitis B or C is considered necessary. Immune reactivation syndrome: In HIV-infected patients with pre-existing severe immune deficiency, typically in the first few weeks or months after initiation of combination ART, an inflammatory reaction to asymptomatic or residual opportunistic pathogens (e.g., CMV retinitis, mycobacterial infections, Pneumocystis pneumonia) may arise and cause serious clinical conditions or aggravation of symptoms. Treatment should be instituted when necessary.
Osteonecrosis: Cases of osteonecrosis have been reported particularly in patients with advanced HIV-disease and/or long-term exposure to combination ART. Other causative factors may include corticosteroid use, alcohol consumption, severe immunosuppression, and higher body mass index. Advise patients to seek medical advice if they experience joint aches and pain, joint stiffness or difficulty in movement.
Opportunistic infections: The use of antiretroviral therapies including abacavir may lead to development of opportunistic infections. Patients should be closely monitored.
Transmission: Current antiretroviral therapies including abacavir, do not prevent the risk of transmission of HIV to others through sexual contact or contamination with blood. Patients should be counselled on appropriate precautions.
Pregnancy and Lactation
Recommendations on the use of Abacavir in Pregnancy & Lactation Pregnancy:
Abacavir crosses the placenta in relatively large amount (Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission, n.d.). Abacavir is a nucleoside reverse transcriptase inhibitor (NRTI) and like other NRTI, mitochondria dysfunction has been reported in infants exposed to abacavir in utero. This presents as haematological, metabolic and neurological disorders
Abacavir has been reported to have the ability of inducing skeletal abnormalities when given to rats at a dose 35 times the recommended clinical daily dosage. This effect was not found at a dose 9 times the recommended daily dose. However, there has been no documented serious signs of teratogenic or fetotoxic damages in humans at the recommended dosage.
Lactation:
There is currently poor experience and scanty report on use of abacavir during lactation. Abacavir is secreted in breastmilk, though, in small quantity which some has reported as not enough to cause any side effects in the infant. Also, worth noting is that the safety and effectiveness of ABC have been established children aged 3 months and older and is a component of the preferred first line in children less than 3 years of age Recommendations
The efficacy and safety of combined therapy with abacavir in preventing vertical transmission of HIV to the newborn are not well known. Therefore, it is currently not among the recommended first- and second-lines antiretroviral drugs in pregnancy and lactation according to the national guidelines. However, if indicated, it should not be withheld in pregnancy (with the possible exception of the 1st trimester) because the expected benefit to the HIV-positive mother probably outweighs the unknown risk to the foetus.
Interactions of Ziagen with Other Drugs
Ethanol: Alcohol intake may increase exposure to abacavir, but clinical significance is unknown. Abacavir has no effect on the metabolism of ethanol Methadone: Oral clearance of methadone may be increased with co-administration of abacavir. Reduced plasma levels of methadone may lead to withdrawal symptoms (Close monitoring recommended). Ribavirin: Co-administration of ribavirin and abacavir has been associated with reduced response rate to hepatitis C therapy that contains ribavirin (Consider substituting abacavir for another NRTI).
Side effects of Ziagen
Life-threatening hypersensitivity reactions can occur in 3 – 9% of patients (see under Pharmacovigilance). Common side effects may include rash, nausea, vomiting, diarrhoea, abdominal pain, anorexia; lethargy, fatigue, fever; dyspnoea, cough; headache, insomnia, dizziness; blood disorders; lipodystrophy (see Therapeutic Notes); pancreatitis, liver damage and lactic acidosis with or without hepatic steatosis (see hepatic disease); very rarely Stevens-Johnson syndrome and toxic epidermal necrolysis. Rash and GI disturbances more common in children.
Chemical Structure of Ziagen